Notes on the dish soap protocol
- olivertburton

- Dec 16, 2025
- 8 min read
Recently we published this protocol using dish soap as a key ingredient for intracellular flow cytometry assays. The aim today is to provide a bit of clarification on this and to address a concern that has been repeatedly raised.
To start, let's clarify what the protocol is for. This is meant to help when you need to combine staining targeting molecules in different cellular compartments. Why do we need this? This is primarily an issue when we want to detect things in the cytoplasm, like fluorescent protein reporters or cytokines, but we also want access to the nucleus for transcription factors or DNA. Retention of cytoplasmic fluorescent reporters such as GFP can be really problematic with intracellular staining. Fix-perm kits targeting the nucleus usually destroy GFP signals because the detergents punch big holes in the plasma membrane and GFP is relatively small. For a weak signal such as the iCas9 reporter here, we can easily lose all the fluorescence if we try to stain for a transcription factor such as Foxp3. The two approaches aren’t really compatible.
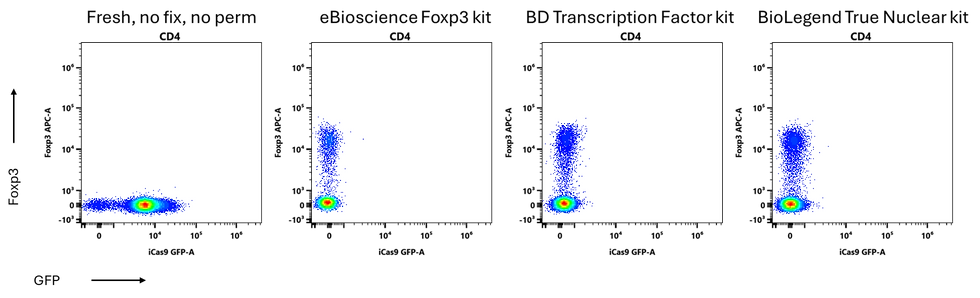
There are a couple of published protocols that are meant to allow you to co-stain for proteins in the nucleus, such as transcription factors like Foxp3, while also preserving fluorescent proteins like GFP. They don’t really work properly in my hands. They preserve some of the GFP, but when we look at the transcription factor staining, it’s really poor.
Here I have a surface-expressed Thy1.1 that faithfully reports Foxp3 expression. We can see that it correlates really nicely with Foxp3 staining when we use a transcription factor staining kit. This is our ground truth.

The published protocols fail to detect most of the Tregs.
We have the same problem with cytokines as we do with GFP. These are also often in the cytoplasm or little granules, and can be lost when you use a transcription factor staining kit.
Here I’m showing you IL-4 staining on mouse CD4 T cells. With the dedicated cytokine staining kit, BioLegend’s CytoFast, we get some IL-4. When we use the transcription factor kits, it’s pretty much the same as if we hadn’t fixed at all.

This is effect varies by cytokine. TNF can be membrane-bound, so it is retained better. IL-4, IL-2 and IL-10 are often lost, while IFNg and IL-17 are usually preserved adequately.
Here is the dish soap protocol (right hand column):

This protocol is good for when you are trying to combine staining for antigens in different cellular compartments. It is not the single best solution for any one thing. If you just want to detect GFP, you’re better off not fixing. If you just want to stain for transcription factors, use a transcription factor kit. But, if you want a little from column A and a little from column B, this may be the way to go.
Now, what are the concerns about this? These basically boil down to the soap not being a scientific reagent. This is true. Why is this potentially a problem? Well, it's possible that the manufacturer, Proctor & Gamble, could change the recipe without notice. The other concern in this vein is that the Fairy dish soap may not undergo QC like dedicated flow cytometry staining kits. This could mean you'd get batch effects.
My thoughts on this are as follows:
I find it rather incredible that Proctor & Gamble is not performing extensive QC on their products. This is a massive, international company. Yes, that QC will not be dedicated to the use of Fairy in flow cytometry, so point taken there.
What if they dilute or concentrate the Fairy soap and don't tell us? In my testing, I've found that the concentration can be varied at least 2x (and probably 4x) up or down with negligible impact. So, it very likely would not matter.
You only need to buy one bottle of dish soap ever. A single bottle is enough to treat something like half a million samples. You, personally, are not likely to ever experience batch effects with this because you will only ever work with one batch. That is not true for kits. I do recommend testing the concentration 2x up and 2x down with your bottle when you first start using it, just to be sure.
I have been using this approach for 8 years. It has been stable in that time.
Scientific kits have not been stable in that time. At least one kit now gives quite different results to when I first started testing the dish soap as an alternative. I don't recall being notified about that.
I have tested this with three different brandings of "Fairy" in two countries.
Okay, so, as with everything, test it before you use it and ensure that it works for you. At the end of this post, I'm including a shorter version of the protocol for day-to-day use. Check the protocols paper for much more info and tips.
Important point:
Formaldehyde is not the same as paraformaldehyde. The dish soap protocol specifically uses formaldehyde. This is the cheap stuff for histology that comes in big multi-litre plastic bottles. It is methanol-stabilized so it can be stored at room temperature. We get ours from VWR, but any neutral-buffered formalin should work. To clarify, 4% formaldehyde is the same as 10% neutral buffered formalin, which is different to 4% PFA. If you use PFA, you will not get good staining. PFA causes much more crosslinking and appears to limit access to the nucleus, so your transcription factor staining will suck. Also, don't use zinc-buffered formalin.

Limitations:
This protocol doesn't open the nucleus as much as you get with dedicated transcription factor kits or methanol. This means you need to put a little more thought into which fluorophores you use for the transcription factor staining. Using large molecules may limit access of the antibody conjugate to the nucleus. My recommendations are to use Real dyes, Alexa Fluors (or related dyes such as Spark dyes) or anything PE-related. APC is also usually good. Brilliant dyes tend to be lacklustre. See this post on T-bet staining using the BD Cytofix/Cytoperm kit for a guide.
I have not tested this for all antibodies in overnight staining. In general, the closest equivalent is the BD Cytofix/Cytoperm kit. I expect that you will see less epitope retention (poorer staining) than using the Foxp3 kit from eBioscience/ThermoFisher, but far more than using just PFA or formaldehyde. You'll have more cells with the dish soap protocol compared to the Foxp3 kit, so you will need to use more antibody proportionally, perhaps twice as much.
An additional advantage:
Fairy dish soap is green, so you might expect it to add background. At the amount used in the protocol, the solution is colorless (to my eye, anyway). It is also only in contact with the cells for a relatively short time. In contrast, most permeabilization buffers in kits are yellowish, likely due to the use of saponin. I didn't put this in the paper, but you should get lower background (autofluorescence) when using the dish soap protocol. That may help quite a bit depending on which part of the light spectrum you're using. Thanks to Graham Bottley and Mo for reminding me about this.
You also should see greater recovery of cells with this protocol than with transcription factor staining kits. At least 2x, in my experience.
Phospho-flow:
I also want to thank Kevin He for providing a correction/expansion on the failure of the dish soap to work for phospho-flow. He's also pointed out this great page from ThermoFisher that shows which phospho-epitope antibodies work well with which buffers. Basically, the methanol used in standard phospho-flow protocols causes denaturation of the phospho-STAT dimers, allowing antibody access. I wasn't aware of this. This provides an explanation for the dish soap failing to work in this setting, independent of dephosphorylation. I'd tested primarily pSTAT5, pSTAT3 and pERK, all of which only really work with methanol-based approaches, according to Thermo. What I will say is that in my testing, there appears to be an element of dephosphorylation as well. Adding phosphatase inhibitors to the staining buffer did rescue a bit of phospho-STAT5 staining in my tests. It wasn't complete, though, and most of the inhibitors I tried increased the background, reducing resolution. So, if you're doing something other than pSTATs or pERK, the dish soap protocol may work. This area requires further testing.
There was one neat thing that came out of this testing of phosphatase inhibitors, and I didn't include it in the paper because it didn't really fit thematically. If you incubate your cells with orthovanadate before fixation, it appears that you can capture low-level endogenous phosphorylation. What I'm talking about is the level of signalling produced by cells secreting and binding cytokines at steady state without stimulation or addition of high concentrations of exogenous cytokines.
The dish soap protocol
Reagents:
4% Formaldehyde VWR 9713.5000 (5L), neutral buffered
PBS
Fairy dishwashing liquid
--In other countries, this may be Dreft, Dawn, Yes or JAR.
Tween-20
FBS (substitute 0.5% BSA if you prefer)
0.5M EDTA pH 8
Optional: Triton X-100
Prepare pre-dilutions:
5% Tween-20 in PBS
· Dispense 9.5ml PBS into a 15ml Falcon tube.
· Cut the tip of a P1000 (blue) tip with scissors to widen the bore.
· Pipette 500ul of Tween-20 into the 15ml tube
· Cap and invert several times to mix
· Store indefinitely at room temperature
5% Fairy in PBS
· Dispense 9.5ml PBS into a 15ml Falcon tube.
· Cut the tip of a P1000 (blue) tip with scissors to widen the bore.
· Pipette 500ul of Fairy into the 15ml tube
· Cap and invert several times to mix
· Store indefinitely at room temperature
Optional: 5% Triton X-100 in PBS
· Dispense 9.5ml PBS into a 15ml Falcon tube.
· Cut the tip of a P1000 (blue) tip with scissors to widen the bore.
· Pipette 500ul of Triton X-100 into the 15ml tube
· Cap and invert several times to mix
· Store indefinitely at room temperature
Working solutions:
Perm buffer: PBS with 0.05% Fairy
· 9ml PBS
· 100µl 5% Fairy
· Store indefinitely at room temperature
Fixative: 2% formaldehyde with 0.05% Fairy and 0.5% Tween
· 5ml 4% formaldehyde
· 4ml PBS
· 1ml 5% Tween-20
· 100µl 5% Fairy
· Optional: add 200ul 5% Triton X-100 (0.1% final)
· Store indefinitely at room temperature
FACS buffer:
· 1 litre PBS
· 25ml FBS
· 4ml 0.5M EDTA
Protocol:
After surface staining, spin down cells.
Resuspend in 200ul Fixative. Incubate 30min at RT in the dark.
Spin down, remove supernatant.
Resuspend in 100ul Perm buffer. Incubate 15-30min at RT. Blocking may be done at this stage.
Wash twice in FACS buffer.
Stain overnight in FACS buffer at 4C.
Wash twice in FACS buffer.
Notes:
Adding triton allows slightly better transcription factor staining at the expense of a slight loss in fluorescent protein signal. So, this allows you to tune the protocol a bit if you have a strong GFP.
Fixation temperature is another variable that allows tuning for more transcription factor staining with less GFP retention, or vice versa. The best all-purpose approach is to use room temperature (21C), as indicated above. If you drop the temperature of the fixation step, you get less fixation, less GFP is retained, and more transcription factor staining can be achieved. I do not recommend increasing the temperature. The post-fix permeabilization step should always be done at room temperature for best results.
Other dish soaps may work. I have not tested many options. Ecover did not work as well. A good quality generic would probably be fine--generics are often identical, even made on the same conveyer belts in the same factories as the brand name item. I would suggest avoiding any soaps with oxidizing or whitening agents.









Comments